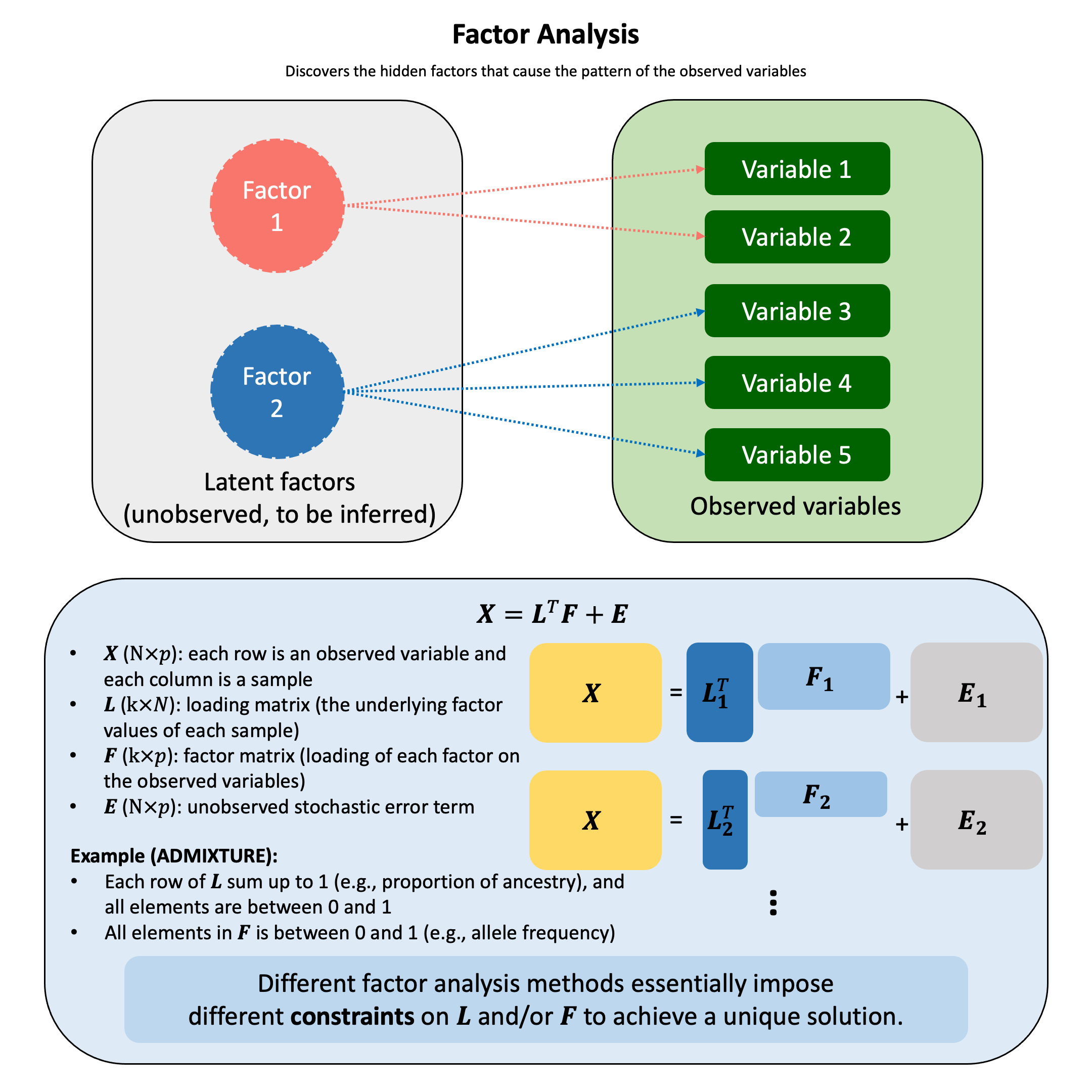
Factor Analysis#
Factor analysis assumes that the observed variables are caused by a smaller number of unobserved (latent) factors and tries to explain why variables are correlated.
Graphical Summary#
Key Formula#
The fundamental factor analysis model:
where:
\(\mathbf{X}\) is a \(N \times p\) matrix of observed variables (\(N\) is the number of samples, \(p\) is the number of variables)
\(\mathbf{L}\) is a \(k \times N\) matrix of loading matrix (loadings of each observation)
\(\mathbf{F}\) is a \(k \times p\) matrix of factor scores (how strongly each variable relates to each factor)
\(\mathbf{E}\) is a \(N \times p\) matrix of unique errors (noise)
\(k \leq p\), \(k\) is the number of latent factors
Technical Details#
Indeterminacy of Matrix Decomposition#
For any invertible matrix \(\mathbf{T}\) (sometimes called rotation matrix):
Result: Infinitely many \((\mathbf{L}, \mathbf{F})\) pairs fit the data equally well (i.e., same \(\mathbf{E}\)). Therefore the key to the factor analysis is the constraints on \(\mathbf{L}\) and/or \(\mathbf{F}\).
Common Constraints#
The estimated factors and loadings must often satisfy domain-specific constraints to ensure they are biologically or statistically meaningful. The specific constraints depend on the application context.
Constraints in Admixture Analysis#
In the admixture analysis, we consider the problem of \(N\) individuals that comes from \(k\) ancestries, and \(p\) is the number of genetic variants.
\(\mathbf{L}\)(\(k \times N\)) is the proportion of each ancestry for each individual; \(\mathbf{F}\) (\(k \times p\)) is allele frequency of each variant in each ancestry.
Constraints:
\(\forall i \in \{1,..., N\}, \sum_j\mathbf{L}_{ij}=1\): only the \(k\) ancestries are considered
\(\forall i \in \{1,..., N\}, j \in \{1,..., p\}, 0 \leq\mathbf{L}_{ij} \leq 1\): the proportions of ancestry for each individual are between 0 and 1
\(\forall i \in \{1,..., N\}, j \in \{1,..., p\}, 0 \leq\mathbf{F}_{ij} \leq 1\): genetic variant frequencies are between 0 and 1
Constraints in Non-negative Matrix Factorization#
Non-negative matrix factorization (NMF or NNMF) is a group of algorithms to factorize a matrix \(\mathbf{X}\) into two matrices \(\mathbf{F}\) and \(\mathbf{L}\), with the property that all three matrices have no negative elements.
This non-negativity makes the resulting matrices easier to inspect, as the factors can often be interpreted as additive parts.
Constraints in Principal Component Analysis#
PCA can also be considered as a factor analysis, which we will introduce more in Lecture: principal component analysis. It is closely related to the singular value decomposition of a matrix.
Proportion of Variance Explained#
The total variance in \(\mathbf{X}\) can be decomposed into explained and unexplained components:
A good factor analysis should:
Explain most of the variance: This means the error matrix \(\mathbf{E}\) is small relative to the signal \(\mathbf{L}^T\mathbf{F}\)
Easy to interpret the factors: Reveal underlying biological pathways or mechanisms
Use fewer factors than variables: \(k \ll p\)
Achieve dimensionality reduction (fewer latent causes than observed effects)
Example: 100 genetic markers → 2 ancestral populations
Example#
Example 1 – Admixture Analysis using ADMIXTURE#
Human populations have genetic diversity shaped by evolutionary history and migration patterns. Different populations often carry different genetic variants at various frequencies due to their geographic origin and ancestry. A key question in population genetics is: given an individual’s genotypes at many genetic markers, can we infer what proportion of their ancestry comes from different ancestral populations? This is particularly important in admixed populations, where individuals have ancestry from multiple sources. Understanding ancestry composition helps us understand human evolution, control for confounding in medical studies, and interpret genetic variation in diverse populations.
Setup#
To study admixture, we first simulate data by mimicking how nature creates admixed individuals.
Allele Frequency Matrix (\(\mathbf{F}\)): Represents the genetic “fingerprints” of ancestral populations. Each entry defines the frequency of a genetic variant within a specific population. We assume these frequencies differ due to geographic isolation and drift.
We start by assuming that two ancestral populations have very distinct marker frequencies. We represent these population-specific allele frequencies in matrix \(\mathbf{F}\), where \(\mathbf{F}[k, j]\) = frequency of marker \(j\) in population \(k\).
The key insight is that the two populations differ substantially at these markers, making them genetically distinguishable.
rm(list=ls())
set.seed(74)
N <- 50 # Number of individuals
p <- 100 # Number of markers (SNPs)
k <- 2 # Number of ancestral populations
# Create L matrix with very distinct population frequencies
F_true <- matrix(0, nrow = k, ncol = p)
# Population 1: generally higher frequencies in the first 50 SNPS, lower frequencies in the last 50
F_true[1, 1:50] <- runif(50, 0.9, 0.95)
F_true[1, 51:100] <- runif(50, 0.05, 0.1)
# Population 2: inverse pattern
F_true[2, 1:50] <- runif(50, 0.05, 0.3)
F_true[2, 51:100] <- runif(50, 0.8, 0.95)
# Add row and column names
colnames(F_true) <- paste0("SNP", 1:p)
rownames(F_true) <- paste0("POP", 1:k)
print("F matrix (first 10 SNPs):")
F_true[,1:10]
[1] "F matrix (first 10 SNPs):"
| SNP1 | SNP2 | SNP3 | SNP4 | SNP5 | SNP6 | SNP7 | SNP8 | SNP9 | SNP10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| POP1 | 0.93523081 | 0.92400022 | 0.90980088 | 0.94684854 | 0.9434915 | 0.9107016 | 0.9012043 | 0.9427398 | 0.9351358 | 0.9478991 |
| POP2 | 0.06645544 | 0.07491498 | 0.07030163 | 0.05800303 | 0.1086709 | 0.1401050 | 0.1588124 | 0.2446992 | 0.2440696 | 0.1840646 |
Ancestry Proportion Matrix (\(\mathbf{L}\)): Represents the “composition” of each individual. For example, an individual’s row might indicate they are 60% Population A and 40% Population B.
Now we create fifty individuals with different ancestry mixtures:
Individuals 1-15: Pure ancestry from Population 1
Individuals 16-30: Pure ancestry from Population 2
Individuals 31-40: Mixed ancestry (80% Population 1, 20% Population 2)
Individuals 41-50: Mixed ancestry (30% Population 1, 70% Population 2)
We represent each individual’s ancestry proportions in matrix \(\mathbf{L}\), where \(\mathbf{L}[k, i]\) = proportion of individual \(i\)’s genome from population \(k\). Each column of \(\mathbf{L}\) must sum to 1, representing 100% ancestry.
transpose_L_true <- matrix(0, nrow = N, ncol = k)
# Individuals 1-15: Pure Pop1
transpose_L_true[1:15, 1] <- 1.0
transpose_L_true[1:15, 2] <- 0.0
# Individuals 16-30: Pure Pop2
transpose_L_true[16:30, 1] <- 0.0
transpose_L_true[16:30, 2] <- 1.0
# Individuals 31-40: Pop1-Pop2 admixture (80-20)
transpose_L_true[31:40, 1] <- 0.8
transpose_L_true[31:40, 2] <- 0.2
# Individuals 41-50: Pop1-Pop2 admixture (30-70)
transpose_L_true[41:50, 1] <- 0.3
transpose_L_true[41:50, 2] <- 0.7
# Add row and column names
rownames(transpose_L_true) <- paste0("IND", 1:N)
colnames(transpose_L_true) <- paste0("POP", 1:k)
Now we simulate the genotype data, which is a function of their specific ancestry mix (\(\mathbf{L}\)) and the global allele frequencies of those ancestors (\(\mathbf{F}\)):
This was done by calculating the expected allele frequency for that individual at each marker as a weighted average:
Then we sample the individual’s genotype from a binomial distribution with this expected frequency. The result is matrix \(\mathbf{X}\) (the genotype matrix), where \(\mathbf{X}[i, j]\) = genotype (0, 1, or 2 copies) of individual \(i\) at marker \(j\).
X_raw <- matrix(0, nrow = N, ncol = p)
for (i in 1:N) {
for (j in 1:p) {
# Expected frequency = weighted average of population frequencies
p_ij <- sum(transpose_L_true[i, ] * F_true[ , j])
# Sample genotype (0, 1, or 2 copies)
X_raw[i,j] <- rbinom(1, size = 2, prob = p_ij)
}
}
# Add row and column names
rownames(X_raw) <- paste0("IND", 1:N)
colnames(X_raw) <- paste0("SNP", 1:p)
print("A few rows for the generated raw genotype matrix X_raw:")
head(X_raw,3)
[1] "A few rows for the generated raw genotype matrix X_raw:"
| SNP1 | SNP2 | SNP3 | SNP4 | SNP5 | SNP6 | SNP7 | SNP8 | SNP9 | SNP10 | ⋯ | SNP91 | SNP92 | SNP93 | SNP94 | SNP95 | SNP96 | SNP97 | SNP98 | SNP99 | SNP100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IND1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | ⋯ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| IND2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ⋯ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IND3 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | ⋯ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Solve for \(\mathbf{L}\) and \(\mathbf{F}\) using ADMIXTURE#
Now comes the interesting part! We observe the genotype matrix \(\mathbf{X}\), but in reality, we don’t know the true \(\mathbf{L}\) and \(\mathbf{F}\) matrices. We need to estimate them from the data using the ADMIXTURE algorithm.
The ADMIXTURE algorithm is based on maximum likelihood estimation. It finds the \(\mathbf{L}\) and \(\mathbf{F}\) matrices that maximize the probability of observing the genotype data \(\mathbf{X}\).
Model Assumptions: The likelihood calculation assumes:
Hardy-Weinberg Equilibrium (HWE) within each ancestral population (see Lecture: Hardy-Weinberg Equilibrium)
Linkage equilibrium between SNPs (no LD considered)
For individual \(i\) at SNP \(j\) with ancestry proportions \(\mathbf{L}_i\) and ancestral allele frequencies \(\mathbf{F}_j\), the probability of observing genotype \(X_{ij}\) depends on the individual’s ancestry-weighted allele frequency: \(p_{ij} = \sum_{k=1}^K L_{ik} F_{jk}\)
Under HWE, the genotype probabilities are determined by this admixed allele frequency.
Constraints:
\(0 \leq \mathbf{F}_{jk} \leq 1\) (allele frequencies must be valid probabilities)
\(L_{ik} \geq 0\) and \(\sum_{k=1}^K L_{ik} = 1\) (ancestry proportions must sum to 1)
Block Relaxation Strategy:
Fix \(\mathbf{F}\), update \(\mathbf{L}\) to maximize likelihood
Fix \(\mathbf{L}\), update \(\mathbf{F}\) to maximize likelihood
Repeat until convergence
# Log-likelihood function
compute_loglik <- function(X, transpose_L, F) {
p <- ncol(X)
N <- nrow(X)
loglik <- 0
for (i in 1:N) {
for (j in 1:p) {
p_ij <- sum(transpose_L[i, ] * F[ , j])
p_ij <- pmax(0.001, pmin(0.999, p_ij))
loglik <- loglik + X[i, j] * log(p_ij) + (2 - X[i, j]) * log(1 - p_ij)
}
}
return(loglik)
}
# EM Algorithm for ADMIXTURE, based on Alexander et al. 2009
# The key is the E-step computes expected counts of alleles FROM EACH POPULATION
admixture_em <- function(X, k, max_iter = 100, tol = 1e-4) {
N <- nrow(X)
p <- ncol(X)
# Initialize transpose_L (L) and F
F <- matrix(runif(k * p, 0.3, 0.7), nrow = k, ncol = p)
transpose_L <- matrix(runif(N * k), nrow = N, ncol = k)
# this is the constraint on transpose_L: each row sums to 1 (ancestry proportions for each individual)
for (i in 1:N) {
transpose_L[i, ] <- transpose_L[i, ] / sum(transpose_L[i, ])
}
loglik_history <- numeric(max_iter)
cat("Running ADMIXTURE (EM algorithm) (only first five iterations)...\n")
cat(sprintf("Iteration | Log-likelihood | Change\n"))
cat(sprintf("-----------------------------------------\n"))
for (iter in 1:max_iter) {
# M-step: Update L (allele frequencies)
for (j in 1:p) {
for (k_idx in 1:k) {
numerator <- 0 # E[alleles from pop k with reference variant at SNP j]
denominator <- 0 # E[total alleles from pop k at SNP j]
for (i in 1:N) {
# Current predicted allele frequency for this individual
p_ij <- sum(transpose_L[i, ] * F[, j])
p_ij <- pmax(0.001, pmin(0.999, p_ij))
weight <- transpose_L[i, k_idx] * F[k_idx, j] / p_ij
# Expected number of alleles from pop k (out of X[j,i] observed)
numerator <- numerator + X[i, j] * weight
denominator <- denominator + 2 * weight
}
if (denominator > 0) {
F[k_idx, j] <- numerator / denominator
}
F[k_idx, j] <- pmax(0.01, pmin(0.99, F[k_idx, j]))
}
}
# M-step: Update F (ancestry proportions)
# CRITICAL: For each individual and population, sum ACROSS ALL SNPS
for (i in 1:N) {
transpose_L_new <- numeric(k)
for (k_idx in 1:k) {
total_k <- 0 # Expected alleles from pop k across all SNPs
for (j in 1:p) {
p_ij <- sum(transpose_L[i, ] * F[ , j])
p_ij <- pmax(0.001, pmin(0.999, p_ij))
# Posterior prob that an observed allele at SNP j came from pop k
weight_allele <- transpose_L[i, k_idx] * F[k_idx, j] / p_ij
# Expected count of alleles from pop k at this SNP
# (out of X[j,i] observed alleles)
total_k <- total_k + X[i, j] * weight_allele
}
transpose_L_new[k_idx] <- total_k
}
# Normalize: total expected alleles across all populations should equal 2*J
total <- sum(transpose_L_new)
if (total > 0) {
transpose_L[i, ] <- transpose_L_new / total
} else {
transpose_L[i, ] <- rep(1/k, k)
}
}
# Compute log-likelihood
loglik <- compute_loglik(X, transpose_L, F)
loglik_history[iter] <- loglik
if (iter == 1) {
cat(sprintf("%9d | %14.2f | %s\n", iter, loglik, "-"))
} else {
if (iter <= 5) {
change <- loglik - loglik_history[iter - 1]
cat(sprintf("%9d | %14.2f | %+.6f\n", iter, loglik, change))
}
if (abs(change) < tol) {
cat(sprintf("\nConverged after %d iterations!\n", iter))
break
}
}
}
return(list(F = F, transpose_L = transpose_L, loglik_history = loglik_history[1:iter]))
}
# Run ADMIXTURE
result <- admixture_em(X_raw, k = 2, max_iter = 100, tol = 1e-4)
F_estimated <- result$F
transpose_L_estimated <- result$transpose_L
Running ADMIXTURE (EM algorithm) (only first five iterations)...
Iteration | Log-likelihood | Change
-----------------------------------------
1 | -6717.24 | -
2 | -6640.32 | +76.911938
3 | -6505.67 | +134.657908
4 | -6286.42 | +219.247961
5 | -5970.80 | +315.618363
Compare Estimated vs True Parameters and Visualize Ancestry Proportions#
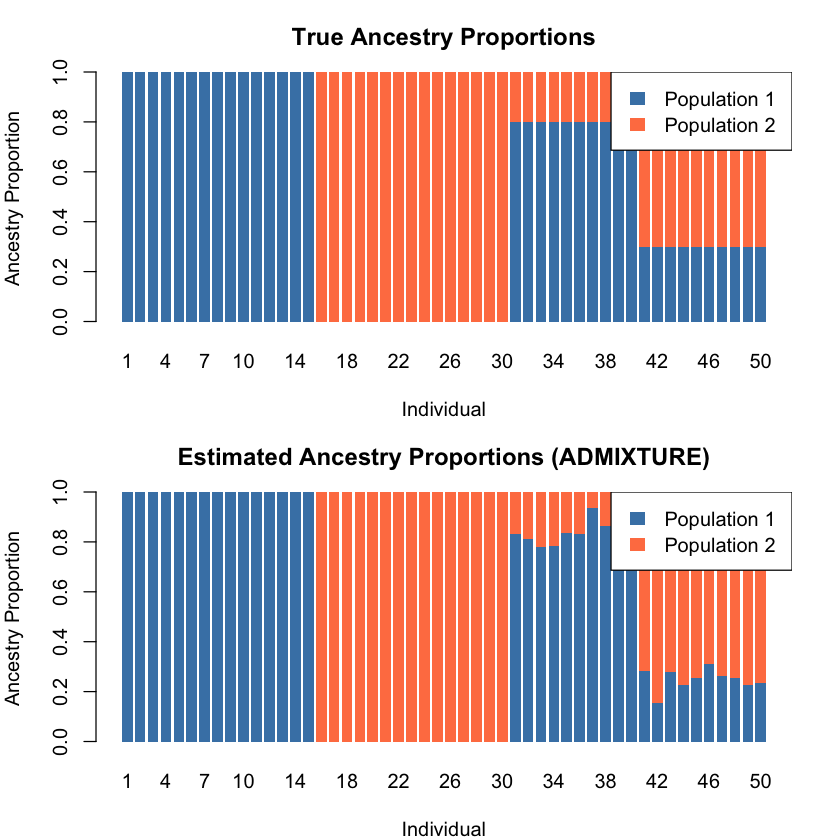
An important issue in mixture model inference is label switching: the optimization algorithm doesn’t inherently assign “Population 1” to a particular cluster—it just identifies the clusters. The populations could be ordered in any way: (1,2) or (2,1) in a two-population case.
cor1 <- cor(transpose_L_estimated[ , 1], transpose_L_true[ , 1])
cor2 <- cor(transpose_L_estimated[ , 2], transpose_L_true[ ,1])
if (cor2 > 0 & cor1 < 0) {
# Labels are switched
transpose_L_estimated <- transpose_L_estimated[ , c(2, 1)]
F_estimated <- F_estimated[c(2, 1), ]
cat("Note: Population labels were switched during estimation (expected behavior)\n\n")
}
par(mfrow = c(2, 1), mar = c(4, 4, 3, 2))
# True ancestry
barplot(t(transpose_L_true), col = c("steelblue", "coral"), border = NA,
main = "True Ancestry Proportions",
xlab = "Individual", ylab = "Ancestry Proportion",
names.arg = 1:N)
legend("topright", legend = c("Population 1", "Population 2"),
fill = c("steelblue", "coral"), border = NA)
# Estimated ancestry
barplot(t(transpose_L_estimated), col = c("steelblue", "coral"), border = NA,
main = "Estimated Ancestry Proportions (ADMIXTURE)",
xlab = "Individual", ylab = "Ancestry Proportion",
names.arg = 1:N)
legend("topright", legend = c("Population 1", "Population 2"),
fill = c("steelblue", "coral"), border = NA)
Example 2 – Exploratory Factor Analysis in Gene Expression#
Suppose you measure expression levels of five genes across 100 tissue samples. You notice that some genes tend to go up or down together—but why? Could it be because they’re controlled by the same underlying biological pathway? In this example, we’ll explore how factor analysis helps us uncover those hidden pathways. We’ll start by letting the data show us what structure exists (exploratory analysis), and then we’ll test a specific theory about which genes belong to which pathways – showing how this detective work can reveal the invisible biological forces driving patterns in your data. Here we perform non-negative matrix factorization on the observed data to reveal the underlying two pathways.
First, let’s create toy data with a known structure where we have two latent biological pathways affecting gene expression.
Setup#
rm(list=ls())
library(NMF)
library(ggplot2)
set.seed(29)
n_samples <- 100 # 100 tissue samples
n_genes <- 5 # 5 genes total
# Two latent factors (biological pathways)
factor1 <- rnorm(n_samples) # Inflammatory pathway activity
factor2 <- rnorm(n_samples) # Metabolic pathway activity
# Create gene expression data
# Genes 1-2: belong to Factor 1 (Inflammatory)
# Genes 3-5: belong to Factor 2 (Metabolic)
expression <- matrix(0, nrow = n_samples, ncol = n_genes)
for (i in 1:n_samples) {
# the exponential function ensures non-negativity in the observed data
expression[i, 1:2] <- exp(0.8 * factor1[i] + rnorm(2, 0, 0.3))
expression[i, 3:5] <- exp(0.5 * factor2[i] + rnorm(3, 0, 0.3))
}
# Name the genes
colnames(expression) <- c(paste0("IL", 1:2), # Inflammatory genes
paste0("METAB", 1:3)) # Metabolic genes
rownames(expression) <- paste0("Sample", 1:n_samples)
cat("Data dimensions:", nrow(expression), "samples x", ncol(expression), "genes\n")
cat("\nFirst few rows of expression data:\n")
round(head(expression), 2)
Data dimensions: 100 samples x 5 genes
First few rows of expression data:
| IL1 | IL2 | METAB1 | METAB2 | METAB3 | |
|---|---|---|---|---|---|
| Sample1 | 0.28 | 0.51 | 1.16 | 0.78 | 0.61 |
| Sample2 | 0.35 | 0.35 | 1.15 | 1.95 | 1.00 |
| Sample3 | 1.64 | 0.95 | 0.31 | 0.42 | 0.60 |
| Sample4 | 2.33 | 3.82 | 0.44 | 0.22 | 0.98 |
| Sample5 | 0.44 | 0.41 | 1.55 | 2.28 | 1.44 |
| Sample6 | 4.68 | 4.98 | 2.93 | 2.16 | 2.58 |
Non-negative Matrix Factorization#
Then we perform the non-negative matrix factorization on the observed expression data.
result <- nmf(expression, rank = 2, method = "brunet")
# Extract factors
L_estimated <- basis(result)
F_estimated <- coef(result)
NFM Results#
Let’s first look at the estimated \(\mathbf{F}\):
F_estimated
| IL1 | IL2 | METAB1 | METAB2 | METAB3 |
|---|---|---|---|---|
| 0.02208169 | 0.03309531 | 0.30621846 | 0.30510774 | 0.30724205 |
| 0.43319431 | 0.42081824 | 0.03526331 | 0.02846171 | 0.05062435 |
Three genes (METAB1, METAB2, METAB3) contribute to the majority of the first factor (first row in F_estimated); while the other two genes contribute to the second factor.
We can also check the correlation between the estimated factor scores and the true factor scores, both are high:
cor(L_estimated[,1], factor2)
cor(L_estimated[,2], factor1)
Reconstruction Based on Estimated \(\mathbf{L}\) and \(\mathbf{F}\) and Visualization#
We can also reconstruct the expression matrix based on the estimated \(\mathbf{L}\) and \(\mathbf{F}\) and compute the variance explained by the model:
# Reconstructed matrix
expression_hat <- fitted(result)
residual <- expression - expression_hat
ss_total <- sum((expression - mean(expression))^2)
ss_resid <- sum((expression - expression_hat)^2)
R2 <- 1 - ss_resid / ss_total
cat("Proportion of variance explained (R-squared):", round(R2, 4), "\n")
Proportion of variance explained (R-squared): 0.8894
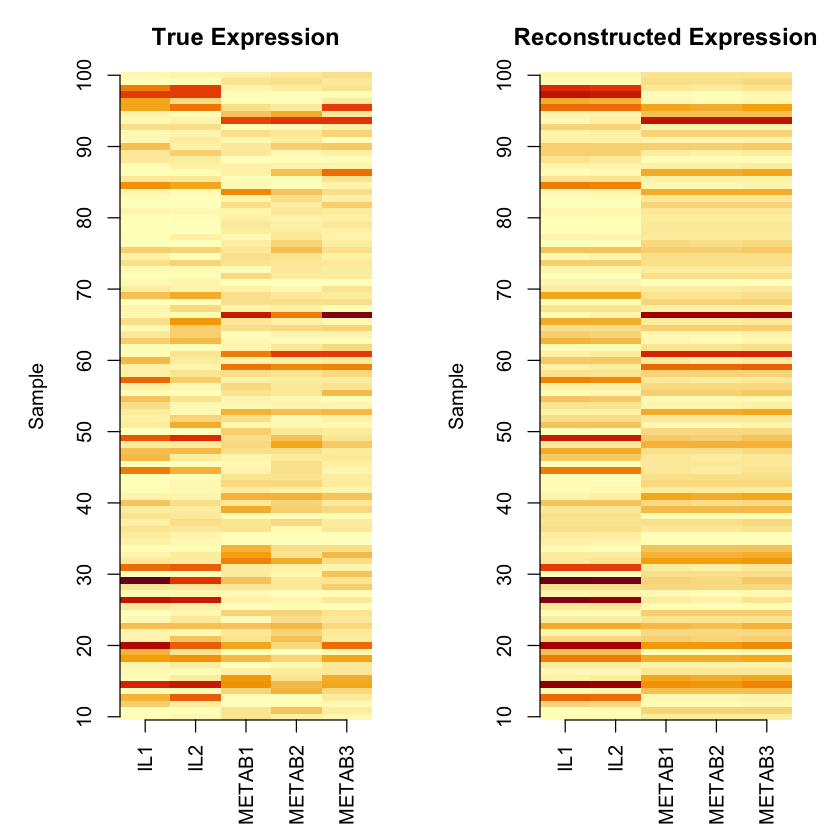
The reconstructed expression data (based on the estimated \(\mathbf{L}\) and \(\mathbf{F}\)) aligns well with the measured expression data:
# Create data frame with gene labels
df <- data.frame(
true = as.vector(expression),
reconstructed = as.vector(expression_hat),
gene = rep(colnames(expression), each = n_samples)
)
# Calculate correlation
cor_val <- cor(df$true, df$reconstructed)
# Create plot with colors by gene
ggplot(df, aes(x = true, y = reconstructed, color = gene)) +
geom_point(alpha = 0.6, size = 2) +
geom_abline(intercept = 0, slope = 1, color = "black",
linetype = "dashed", linewidth = 1) +
annotate("text", x = 3.5, y = 6.3,
label = paste("Correlation =", round(cor_val, 4)),
color = "black", size = 5) +
scale_x_continuous(breaks = 0:7, limits = c(0, 7), expand = c(0, 0)) +
scale_y_continuous(breaks = 0:7, limits = c(0, 7), expand = c(0, 0)) +
coord_fixed(ratio = 1) + # Makes it square
labs(x = "True Expression",
y = "Reconstructed Expression",
title = "True vs Reconstructed Expression",
color = "Gene") +
theme_bw() +
theme(panel.grid.minor = element_blank())
par(mfrow = c(1, 2), mar = c(5, 5, 3, 2))
# True expression heatmap
image(t(expression),
main = "True Expression",
ylab = "Sample",
col = hcl.colors(50, "YlOrRd", rev = TRUE),
axes = FALSE)
axis(1, at = seq(0, 1, length.out = n_genes), labels = colnames(expression), las = 2)
axis(2, at = seq(0, 1, length.out = 10), labels = seq(10, 100, by = 10))
# Reconstructed expression heatmap
image(t(expression_hat),
main = "Reconstructed Expression",
ylab = "Sample",
col = hcl.colors(50, "YlOrRd", rev = TRUE),
axes = FALSE)
axis(1, at = seq(0, 1, length.out = n_genes), labels = colnames(expression), las = 2)
axis(2, at = seq(0, 1, length.out = 10), labels = seq(10, 100, by = 10))